A solid can be defined as a substance which exists in the solid-state, which is one of the four fundamental states of matter. Solids feature closely packed atoms whose kinetic energies are much lower than those of liquids and gases. OR What is solid and examples? A solid is that state of matter which has a fixed shape, mass, and volume. It shows very small changes in volume by changing the temperature. It can not be compressed, e.g. — Sand, Wood, Copper, Ice, etc.More
Note:The elements which are present in Maroon color box are Solid State.

| 1 H Hydrogen | 2 He Helium | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 li lithium | 4 Be Beryllium | 5 B Boron | 6 C Carbon | 7 N Nitrogen | 8 O Oxygen | 9 F Fluorine | 10 Ne Neon | ||||||||||
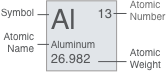
| 11 Na sodium | 12 Mg Magnesium | 13 Al Aluminium | 14 Si Silicon | 15 P Phosphorus | 16 S Sulfur | 17 Cl Chlorine | 18 Ar Argon | ||||||||||
| 19 K Potassium | 20 Ca Calcium | 21 Sc Scandium | 22 Ti Titanium | 23 V Vanadium | 24 Cr Cromium | 25 Mn Manganesse | 26 Fe Iron | 27 Co Cobalt | 28 Ni Nickel | 29 Cu Copper | 30 Zn Zinc | 31 Ga Gallium | 32 Ge Germanium | 33 Ar Arsenic | 34 Se Selanium | 35 Br Bromine | 36 Kr Krypton |
| 37 Rb Rubidium | 38 Sr Strontium | 39 Ca Yttrium | 40 Zr Zirconium | 41 Nb Niobium | 42 Mo Molybdenum | 43 Tc Tecnetium | 44 Ru Ruthenium | 45 Rh Rhodium | 46 Pd Palladium | 47 Ag Silver | 48 Cd Cadnium | 49 In Indium | 50 Sn Tin | 51 Sb Antimony | 52 Te Tellurium | 53 I Iodine | 54 Xe Xenon |
| 55 Cs Caesium | 56 Ba Barium | 57 la Lanthan... | 72 Hf Hafnium | 73 Ta Tantalum | 74 W Tungsten | 75 Re Rhenium | 76 Os Osmium | 77 Ir Iridium | 78 Pt Platinum | 79 Au Gold | 80 Hg Mercury | 81 Tl Thallium | 82 Pb Lead | 83 Bi Bismuth | 84 Po Polonnium | 85 At Astatine | 86 Rn Radon |
| 87 Fr Francium | 88 Ra Radium | 89 Ac Actinium | 104 Rf Rutherfo.. | 105 Db Dubnium | 106 Sg Seaborgium | 107 Bh Bohrium | 108 Hs Hassiumy | 109 Mt Meitnerium | 110 Ds Damstadium | 111 Rg Roentgenium | 112 Cn Copemicium | 113 Nh Nihonium | 114 Fl Flerovium | 115 Mc Moscovium | 116 Lv Livermorium | 117 Ts Tennessi.. | 118 Og Oganesson |
| 58 Ce Cerium | 59 Pr Praseodium | 60 Nd Neodymium | 61 Pm Promethium | 62 Sm Samarium | 63 Eu Europium | 64 Gd Gadolini.. | 65 Tb Terbium | 66 Dy Dysprosium | 67 Ho Holmium | 68 Er Erbium | 69 Tm Thulium | 70 Yb Ytterbium | 71 Lu Lutetium | ||||
| 90 Th Thorium | 91 Pa Protactinium | 92 U Uranium | 93 Np Neptunium | 94 Pu Plutonium | 95 Am Americium | 96 Cm Curium | 97 Bk Berkelium | 98 Cf Californi.. | 99 Es Einstenium | 100 Fm Fermium | 101 Md Mendelevium | 102 No Nobelium | 103 Lr Lawrencium |
Solids have a fixed shape and volume. They are not compressible because their particles are tightly arranged together. They also have a fixed position, and can only vibrate in place. Solids usually have strong intermolecular forces, and they can be classified as either Crystalline or Amorphous solids.