Metals are elements on the periodic table that are malleable, lose electrons easily, good conductors of heat or electricity, and typically appear reflective. Learn about the groupings of metals and nonmetals on the periodic table, features in metallic bonding and reactivity, and their formation of ionic compounds.More
Note:The elements which are present in Orange color box Metals.

| 1 H Hydrogen | 2 He Helium | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 li lithium | 4 Be Beryllium | 5 B Boron | 6 C Carbon | 7 N Nitrogen | 8 O Oxygen | 9 F Fluorine | 10 Ne Neon | ||||||||||
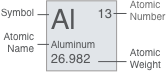
| 11 Na sodium | 12 Mg Magnesium | 13 Al Aluminium | 14 Si Silicon | 15 P Phosphorus | 16 S Sulfur | 17 Cl Chlorine | 18 Ar Argon | ||||||||||
| 19 K Potassium | 20 Ca Calcium | 21 Sc Scandium | 22 Ti Titanium | 23 V Vanadium | 24 Cr Cromium | 25 Mn Manganesse | 26 Fe Iron | 27 Co Cobalt | 28 Ni Nickel | 29 Cu Copper | 30 Zn Zinc | 31 Ga Gallium | 32 Ge Germanium | 33 Ar Arsenic | 34 Se Selanium | 35 Br Bromine | 36 Kr Krypton |
| 37 Rb Rubidium | 38 Sr Strontium | 39 Ca Yttrium | 40 Zr Zirconium | 41 Nb Niobium | 42 Mo Molybdenum | 43 Tc Tecnetium | 44 Ru Ruthenium | 45 Rh Rhodium | 46 Pd Palladium | 47 Ag Silver | 48 Cd Cadnium | 49 In Indium | 50 Sn Tin | 51 Sb Antimony | 52 Te Tellurium | 53 I Iodine | 54 Xe Xenon |
| 55 Cs Caesium | 56 Ba Barium | 57 la Lanthan... | 72 Hf Hafnium | 73 Ta Tantalum | 74 W Tungsten | 75 Re Rhenium | 76 Os Osmium | 77 Ir Iridium | 78 Pt Platinum | 79 Au Gold | 80 Hg Mercury | 81 Tl Thallium | 82 Pb Lead | 83 Bi Bismuth | 84 Po Polonnium | 85 At Astatine | 86 Rn Radon |
| 87 Fr Francium | 88 Ra Radium | 89 Ac Actinium | 104 Rf Rutherfo.. | 105 Db Dubnium | 106 Sg Seaborgium | 107 Bh Bohrium | 108 Hs Hassiumy | 109 Mt Meitnerium | 110 Ds Damstadium | 111 Rg Roentgenium | 112 Cn Copemicium | 113 Nh Nihonium | 114 Fl Flerovium | 115 Mc Moscovium | 116 Lv Livermorium | 117 Ts Tennessi.. | 118 Og Oganesson |
| 58 Ce Cerium | 59 Pr Praseodium | 60 Nd Neodymium | 61 Pm Promethium | 62 Sm Samarium | 63 Eu Europium | 64 Gd Gadolini.. | 65 Tb Terbium | 66 Dy Dysprosium | 67 Ho Holmium | 68 Er Erbium | 69 Tm Thulium | 70 Yb Ytterbium | 71 Lu Lutetium | ||||
| 90 Th Thorium | 91 Pa Protactinium | 92 U Uranium | 93 Np Neptunium | 94 Pu Plutonium | 95 Am Americium | 96 Cm Curium | 97 Bk Berkelium | 98 Cf Californi.. | 99 Es Einstenium | 100 Fm Fermium | 101 Md Mendelevium | 102 No Nobelium | 103 Lr Lawrencium |
Metals are elements that lose electrons easily, that are lustrous (reflective), malleable (can be molded into other shapes), and are good conductors of heat and electricity. Metals are crucial to our ways of life. Not only are they part of our structures and technology, but they are important to the manufacturing of nearly all items. A quick survey of our surroundings yields a variety of metals with important roles. What we don't always notice though, is that our bodies are also dependent on metals. Glancing at the nutrition label of a multivitamin, you'll see dozens of metals listed. Maybe you didn't know that sodium, calcium, magnesium, and zinc are all metals. These metals are necessary for life, and if they're missing in our bodies, our health can be severely compromised. For example, calcium is necessary for healthy bones and magnesium for metabolism. Zinc boosts immune system function, and iron helps our blood cells carry oxygen around the body. However, the metals in our bodies are different from the metal in a spoon or a steel bridge, in that they have lost electrons. They are called cations (more on this later). Metals also have antibiotic properties, which is why railings and handles in public areas are often made of metal. Some orthotics are even made with silver to prevent bacterial growth. Artificial joints are made from titanium alloys that simultaneously prevent infection and make the recipients stronger.
The majority of elements on the periodic table are metals. This periodic table groups elements according to type: metal (blue), nonmetal (yellow), or metalloid (red).All of the metals are grouped together on the left side of the periodic table. Notice that hydrogen, colored red, is grouped with the metals in the top left corner. Even though it is grouped with the metals, hydrogen is considered to be a nonmetal. Some scientists theorize however, that there could be metallic hydrogen in the core of the planet Jupiter.
Many of a metal's remarkable and useful qualities stem from the way that metal atoms bond with each other, known as metallic bonding. Metallic bonding is how metal atoms interact on the atomic level; it is how metal atoms connect to make larger metal structures. Any sample of metal you see, from the metal in your car to the coins in your pocket, is engaging in metallic bonding.