Halogens are the non-metallic elements found in group 17 of the periodic table: and include fluorine, chlorine, bromine, iodine, and astatine. They are the only group whose elements at room temperature include solid, liquid, and gas forms of matter. When halogens react with metals, they produce a range of useful salts, including calcium fluoride, sodium chloride, silver bromide, and potassium iodide. Since halogens are one electron short of having full shells, they can combine with many different elements. They are highly reactive and can be lethal in concentrated amounts. Commercially, halogens are used in disinfectants, lighting, and drug components.More
Note:The elements which are present in Parrot Green color box are Halogens.

| 1 H Hydrogen | 2 He Helium | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 li lithium | 4 Be Beryllium | 5 B Boron | 6 C Carbon | 7 N Nitrogen | 8 O Oxygen | 9 F Fluorine | 10 Ne Neon | ||||||||||
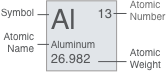
| 11 Na sodium | 12 Mg Magnesium | 13 Al Aluminium | 14 Si Silicon | 15 P Phosphorus | 16 S Sulfur | 17 Cl Chlorine | 18 Ar Argon | ||||||||||
| 19 K Potassium | 20 Ca Calcium | 21 Sc Scandium | 22 Ti Titanium | 23 V Vanadium | 24 Cr Cromium | 25 Mn Manganesse | 26 Fe Iron | 27 Co Cobalt | 28 Ni Nickel | 29 Cu Copper | 30 Zn Zinc | 31 Ga Gallium | 32 Ge Germanium | 33 Ar Arsenic | 34 Se Selanium | 35 Br Bromine | 36 Kr Krypton |
| 37 Rb Rubidium | 38 Sr Strontium | 39 Ca Yttrium | 40 Zr Zirconium | 41 Nb Niobium | 42 Mo Molybdenum | 43 Tc Tecnetium | 44 Ru Ruthenium | 45 Rh Rhodium | 46 Pd Palladium | 47 Ag Silver | 48 Cd Cadnium | 49 In Indium | 50 Sn Tin | 51 Sb Antimony | 52 Te Tellurium | 53 I Iodine | 54 Xe Xenon |
| 55 Cs Caesium | 56 Ba Barium | 57 la Lanthan... | 72 Hf Hafnium | 73 Ta Tantalum | 74 W Tungsten | 75 Re Rhenium | 76 Os Osmium | 77 Ir Iridium | 78 Pt Platinum | 79 Au Gold | 80 Hg Mercury | 81 Tl Thallium | 82 Pb Lead | 83 Bi Bismuth | 84 Po Polonnium | 85 At Astatine | 86 Rn Radon |
| 87 Fr Francium | 88 Ra Radium | 89 Ac Actinium | 104 Rf Rutherfo.. | 105 Db Dubnium | 106 Sg Seaborgium | 107 Bh Bohrium | 108 Hs Hassiumy | 109 Mt Meitnerium | 110 Ds Damstadium | 111 Rg Roentgenium | 112 Cn Copemicium | 113 Nh Nihonium | 114 Fl Flerovium | 115 Mc Moscovium | 116 Lv Livermorium | 117 Ts Tennessi.. | 118 Og Oganesson |
| 58 Ce Cerium | 59 Pr Praseodium | 60 Nd Neodymium | 61 Pm Promethium | 62 Sm Samarium | 63 Eu Europium | 64 Gd Gadolini.. | 65 Tb Terbium | 66 Dy Dysprosium | 67 Ho Holmium | 68 Er Erbium | 69 Tm Thulium | 70 Yb Ytterbium | 71 Lu Lutetium | ||||
| 90 Th Thorium | 91 Pa Protactinium | 92 U Uranium | 93 Np Neptunium | 94 Pu Plutonium | 95 Am Americium | 96 Cm Curium | 97 Bk Berkelium | 98 Cf Californi.. | 99 Es Einstenium | 100 Fm Fermium | 101 Md Mendelevium | 102 No Nobelium | 103 Lr Lawrencium |
The word "halogen" means "salt former" (or "salt maker"). When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide.[5] The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure. All of the halogens form acids when bonded to hydrogen. Most halogens are typically produced from minerals or salts. The middle halogens—chlorine, bromine, and iodine—are often used as disinfectants. Organobromides are the most important class of flame retardants, while elemental halogens are dangerous and can be toxic.