Lanthanides make up the 15 metallic chemical elements with atomic numbers 57 through 71. Called lanthanides because they are chemically similar to lanthanum, these elements and the actinides form the category of rare earth elements. Despite this moniker, these chemicals are fairly abundant in the Earth’s crust. For example, cerium is the 25th most abundant element. Lanthanides oxidize rapidly in moist air, dissolve quickly in acids, and react slowly with oxygen at room temperature. These elements are used in superconductors and hybrid car components, primarily as magnets and batteries. They are also used in the production of specialty glass.More
Note:The elements which are present in Green color box are Lanthanides.

| 1 H Hydrogen | 2 He Helium | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 li lithium | 4 Be Beryllium | 5 B Boron | 6 C Carbon | 7 N Nitrogen | 8 O Oxygen | 9 F Fluorine | 10 Ne Neon | ||||||||||
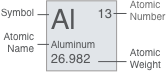
| 11 Na sodium | 12 Mg Magnesium | 13 Al Aluminium | 14 Si Silicon | 15 P Phosphorus | 16 S Sulfur | 17 Cl Chlorine | 18 Ar Argon | ||||||||||
| 19 K Potassium | 20 Ca Calcium | 21 Sc Scandium | 22 Ti Titanium | 23 V Vanadium | 24 Cr Cromium | 25 Mn Manganesse | 26 Fe Iron | 27 Co Cobalt | 28 Ni Nickel | 29 Cu Copper | 30 Zn Zinc | 31 Ga Gallium | 32 Ge Germanium | 33 Ar Arsenic | 34 Se Selanium | 35 Br Bromine | 36 Kr Krypton |
| 37 Rb Rubidium | 38 Sr Strontium | 39 Ca Yttrium | 40 Zr Zirconium | 41 Nb Niobium | 42 Mo Molybdenum | 43 Tc Tecnetium | 44 Ru Ruthenium | 45 Rh Rhodium | 46 Pd Palladium | 47 Ag Silver | 48 Cd Cadnium | 49 In Indium | 50 Sn Tin | 51 Sb Antimony | 52 Te Tellurium | 53 I Iodine | 54 Xe Xenon |
| 55 Cs Caesium | 56 Ba Barium | 57 la Lanthan... | 72 Hf Hafnium | 73 Ta Tantalum | 74 W Tungsten | 75 Re Rhenium | 76 Os Osmium | 77 Ir Iridium | 78 Pt Platinum | 79 Au Gold | 80 Hg Mercury | 81 Tl Thallium | 82 Pb Lead | 83 Bi Bismuth | 84 Po Polonnium | 85 At Astatine | 86 Rn Radon |
| 87 Fr Francium | 88 Ra Radium | 89 Ac Actinium | 104 Rf Rutherfo.. | 105 Db Dubnium | 106 Sg Seaborgium | 107 Bh Bohrium | 108 Hs Hassiumy | 109 Mt Meitnerium | 110 Ds Damstadium | 111 Rg Roentgenium | 112 Cn Copemicium | 113 Nh Nihonium | 114 Fl Flerovium | 115 Mc Moscovium | 116 Lv Livermorium | 117 Ts Tennessi.. | 118 Og Oganesson |
| 58 Ce Cerium | 59 Pr Praseodium | 60 Nd Neodymium | 61 Pm Promethium | 62 Sm Samarium | 63 Eu Europium | 64 Gd Gadolini.. | 65 Tb Terbium | 66 Dy Dysprosium | 67 Ho Holmium | 68 Er Erbium | 69 Tm Thulium | 70 Yb Ytterbium | 71 Lu Lutetium | ||||
| 90 Th Thorium | 91 Pa Protactinium | 92 U Uranium | 93 Np Neptunium | 94 Pu Plutonium | 95 Am Americium | 96 Cm Curium | 97 Bk Berkelium | 98 Cf Californi.. | 99 Es Einstenium | 100 Fm Fermium | 101 Md Mendelevium | 102 No Nobelium | 103 Lr Lawrencium |
The lanthanide (/ˈlænθənaɪd/) or lanthanoid (/ˈlænθənɔɪd/) series of chemical elements[1] comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium.[2][3][4] These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements or rare-earth metals. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. There is some dispute on whether lanthanum or lutetium is a d-block element, but lutetium is usually considered so by those who study the matter;[5][6] it is included due to its chemical similarities with the other 14.[7] All lanthanide elements form trivalent cations, Ln3+, whose chemistry is largely determined by the ionic radius, which decreases steadily from lanthanum to lutetium. These elements are called lanthanides because the elements in the series are chemically similar to lanthanum. Since "lanthanide" means "like lanthanum", it has been argued that lanthanum cannot logically be a lanthanide, but the International Union of Pure and Applied Chemistry (IUPAC) acknowledges its inclusion based on common usage.[8] In presentations of the periodic table, the f-block elements are customarily shown as two additional rows below the main body of the table.[2] This convention is entirely a matter of aesthetics and formatting practicality; a rarely used wide-formatted periodic table inserts the 4f and 5f series in their proper places, as parts of the table's sixth and seventh rows (periods). The 1985 IUPAC "Red Book" (p. 45) recommends using "lanthanoid" instead of "lanthanide", as the ending "-ide" normally indicates a negative ion. However, owing to wide current use, "lanthanide" is still allowed.